New Tool in the Toolbox: Emily’s Entourage Creates Nonsense Mutation Organoid
Emily’s Entourage has always operated with a sense of urgency and refusal to accept the status quo for Cystic Fibrosis (CF), guided in large part by Emily and her family’s energy and commitment. This resolution has led Emily’s Entourage to be at the forefront of CF advancements. Nowhere has this been more evident recently than in the Entourage’s pioneering participation with organoids.
Earlier this year, Emily became the first CF patient to send her cells from the United States to Amsterdam to be developed into organoids. The cells were sent to the Hubrecht Institute, a lab in Utrecht led by Jeffrey Beekman that is in the vanguard of organoid research.
“What’s most remarkable about our role with organoids is that we just did it entirely on our own,” said Peter Haggie, Science Director at Emily’s Entourage. “We figured out how to get the biopsies and how to send Emily’s cells off.”
Given the promising yet nascent stage of Beekman’s research, there was no road map for how to get Emily’s cells to Dutch soil intact. So, in typical Emily’s Entourage fashion, the organization paved its own way, finding the clinical partners, coordinating with the shipping company to transport Emily’s cells, liaising with the Hubrecht Institute, and serving as the hub keeping the entire project on track.
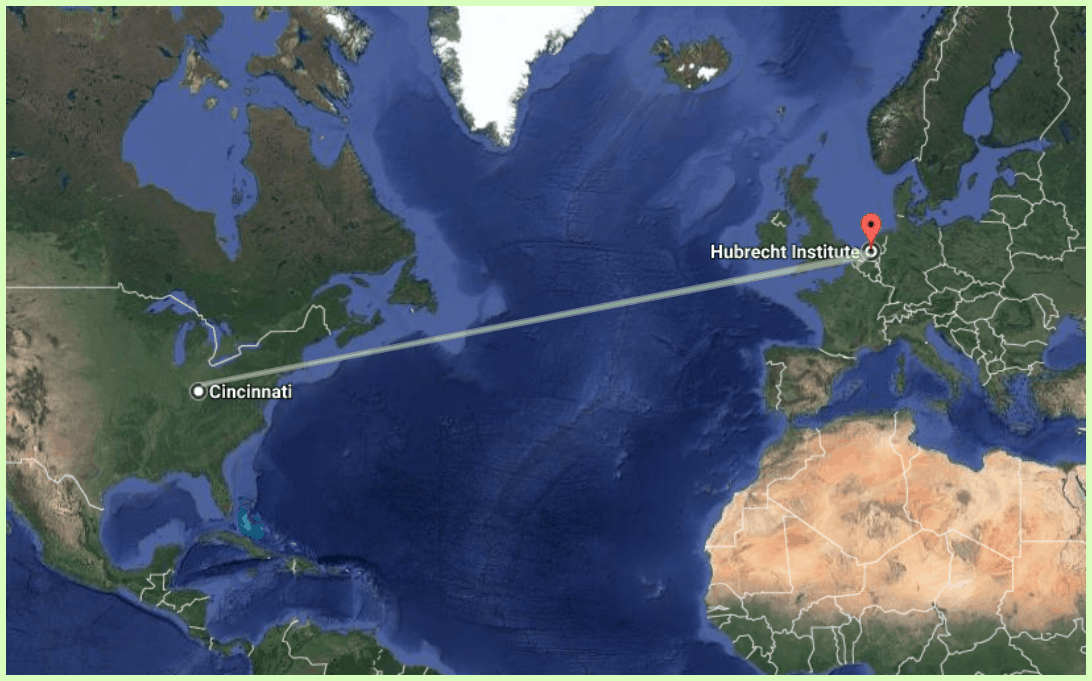
Talk about going the distance! Emily’s cells were transported nearly 4,200 miles to the Hubrecht Institute in Amsterdam, where they were developed into organoids.
Identifying providers with the clinical research infrastructure to biopsy intestinal cells and send them to Hubrecht was the first challenge. The organization found its partners in renowned pulmonologist JP Clancy, MD, and GI surgeon Joe Palermo, MD, at Cincinnati Children’s Hospital.
In March, Emily traveled with her mom Liza to Cincinnati to undergo the procedure, which involved extracting cells from her intestines. While cells from the lungs are most ideal, researchers are using intestinal cells for organoids because the procedure for extracting them is relatively minor and intestinal cells have unique properties that make them “next best” preclinical surrogates for the lungs.

Emily arriving to the William K. Schubert Research Clinic at Cincinnati Children’s.
The brainchild of Beekman, organoids are three-dimensional models developed from cells that express organ features. Because they come from an individual’s cells, they contain the individual’s DNA as well as any defects in it. In Emily’s case, the organoids contain her CF mutations, two copies of the nonsense mutation W1282X. The fact that she has two copies of the same mutation make her cells a particularly valuable research asset.
And what’s so exciting about organoids is that researchers have shown that drug responses in these mini-organs correlate with clinical outcomes in actual patients.
“There’s a tremendous amount of promise with organoids,” said Haggie. “They’re so individualized that we can get a better sense of whether a drug will work on a given patient than ever before.”
This is a profound finding, particularly for those with rare diseases and rare mutations like Emily. It means organoids can be used as a platform for identifying patients with CF mutations that are too rare to qualify for a clinical trial but may benefit from existing therapies, like KALYDECO® and ORKAMBI®, as well as for testing new drugs and combinations of drugs.
Organoids are, in essence, individualized drug testing platforms – personalized medicine at its most promising.
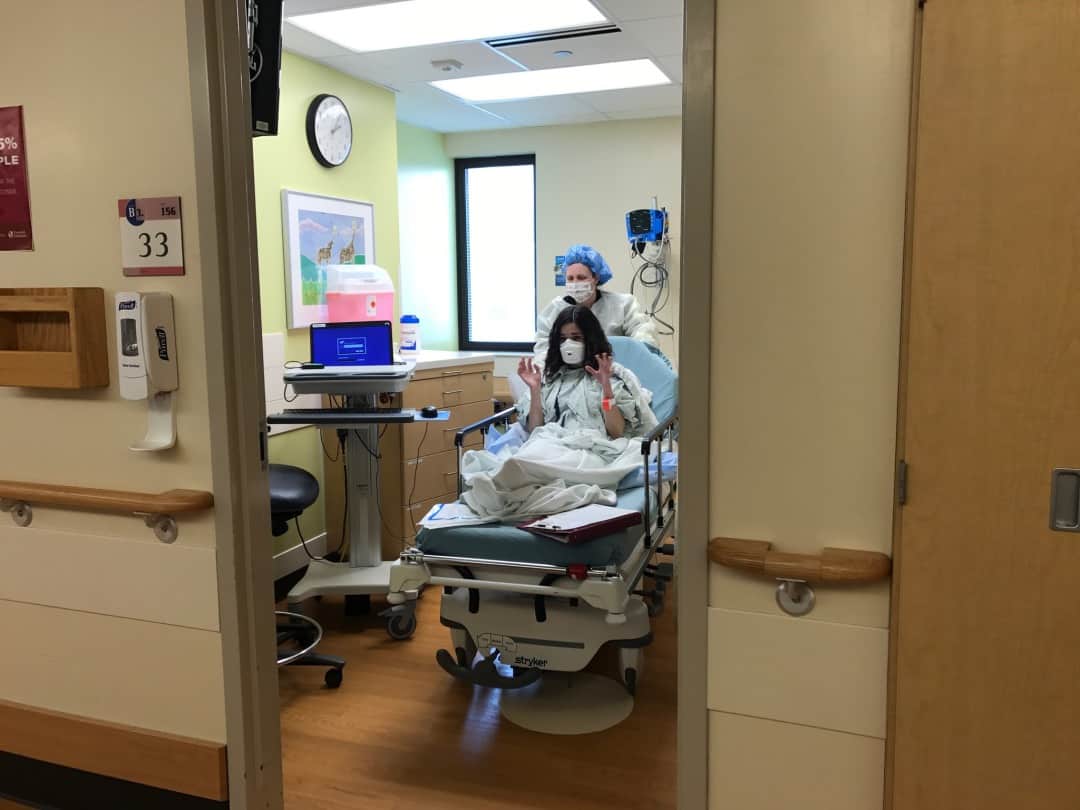
Emily being wheeled away for the procedure to extract intestinal cells.
And the exciting news does not stop there. A recent FDA ruling just upped the ante for the potential transformative role of organoids and other predictive cell models in the treatment of rare diseases. Just last month, the FDA approved the expansion of KALYDECO® to 23 new rare CF mutations exclusively based on in vitro (cell-based) data, not people, as well as the drug’s proven safety record.
The FDA’s decision represents a monumental leap for the CF and rare disease communities. The first time a drug has ever been approved this way, it opens the door to a new era of precision medicine that is no longer entirely dependent on recruiting large disease populations for clinical trials. Testing on cell-based models is cheaper, faster, and far more individualized.
It is too early to know yet, but the early findings suggest that organoids may be poised to become a major player in this new personalized medicine era.
Where promise glistens, Emily’s Entourage quickly follows. No barrier will stand in our way, not even the absence of a roadmap. We are thrilled to have blazed the trail ourselves and to now offer organoids as a new tool in Emily’s Entourage’s ever growing nonsense mutation “toolbox.”
If you are an investigator or company that is interested in obtaining access to these organoids, please contact us here.
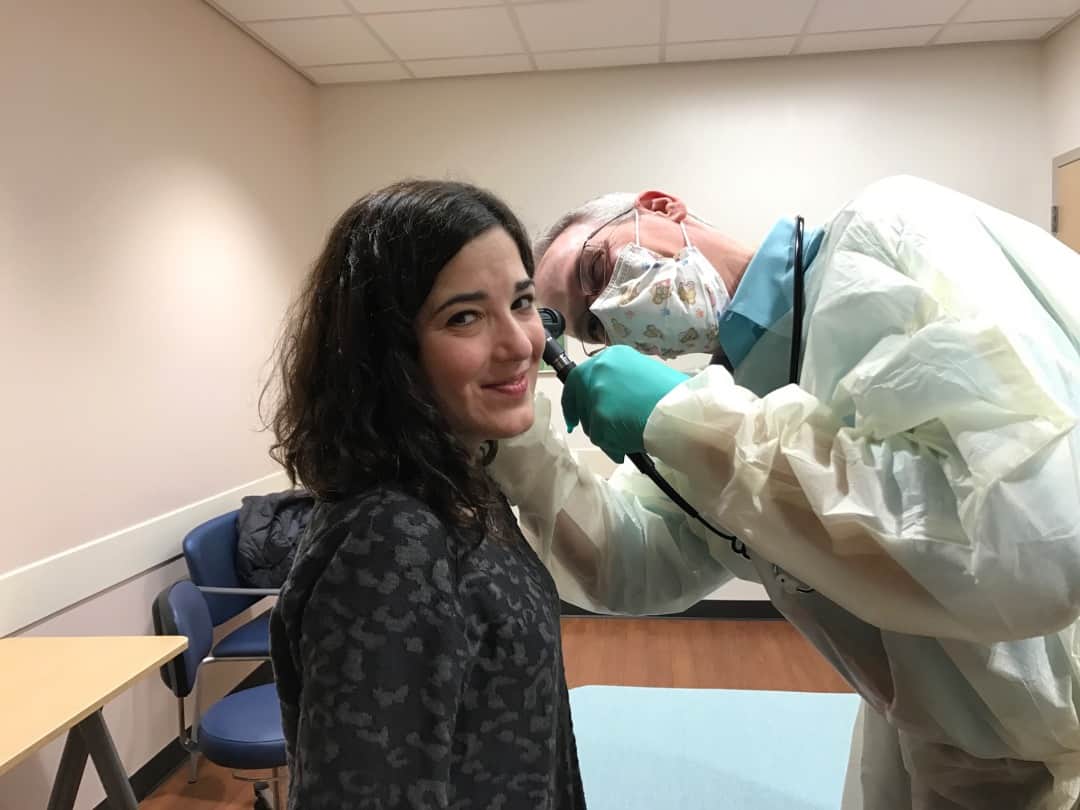
Emily undergoing her pre-operative examination by Dr. Clancy.
