
Advocacy
Spotlighting the Needs of the Final 10%
Advocacy and representation of the final 10% of the CF community is a critical part of EE’s mission. While EE had earned a well-deserved reputation over the years for effective advocacy for research and therapeutic development for those in the final 10% of the CF community, there was a growing realization of its unique value in the regulatory space — and specifically with key regulators like the Food & Drug Administration (FDA).
In 2021, EE brought its advocacy to the FDA by hosting an FDA Patient Listening Session. This was a major step for the organization. EE’s approach to advocacy has always placed the patient in the driver’s seat, working side-by-side with the scientific community. Partnering with the FDA on a session that specifically focused on the unmet needs of the final 10% of the CF community reflected EE’s influential, expanding voice across multiple areas paramount in therapeutic development, including the regulatory space.

FDA Patient Listening Sessions are small, informal meetings, closed to the public, where patient advocacy groups speak with FDA staff about their experience living with and managing a disease. The goals for EE’s listening session were to share and humanize the urgent, unmet therapeutic needs that remain for the final 10% of the CF community; build a strong relationship with the FDA to encourage prioritization and accelerated regulatory reviews and approvals in the future; and ensure the perspectives of the CF community are included at every stage of the drug development and approval processes.
At the meeting, EE educated FDA staff about EE, CF, and the remaining 10% of the CF community that does not benefit from currently available CFTR modulators, including those with CF nonsense mutations. The event included remarks from a clinician as well as six CF community members, including individuals with CF, parents and siblings of individuals with CF, and a parent of a deceased individual with CF, who shared their lived experience with the disease. These deeply personal and heartfelt stories helped convey to the FDA the urgency around seeking treatments for the remaining 10% of the CF community.
Stories highlighted in the FDA listening sessions:
As part of the FDA listening session, participants heard from six members of the CF community, including Abhijit Tirumala, a 19-year-old college student with cystic fibrosis. As part of his testimony, Abhijit shared how CF affects his mental health and how his life would change if a new drug were able to reduce his reliance on daily treatments and medications.

In June 2021, EE conducted its inaugural comprehensive, global survey of the roughly 10% of individuals in the CF community that do not benefit from currently available CFTR modulators due to ineligible mutations, side effects, or lack of access. The purpose of the “Final 10%” survey was to collect information about the health status, impact of CF, unmet treatment needs, and clinical research preferences of those not benefitting from CFTR modulators and present preliminary data and results at the FDA Listening Session.
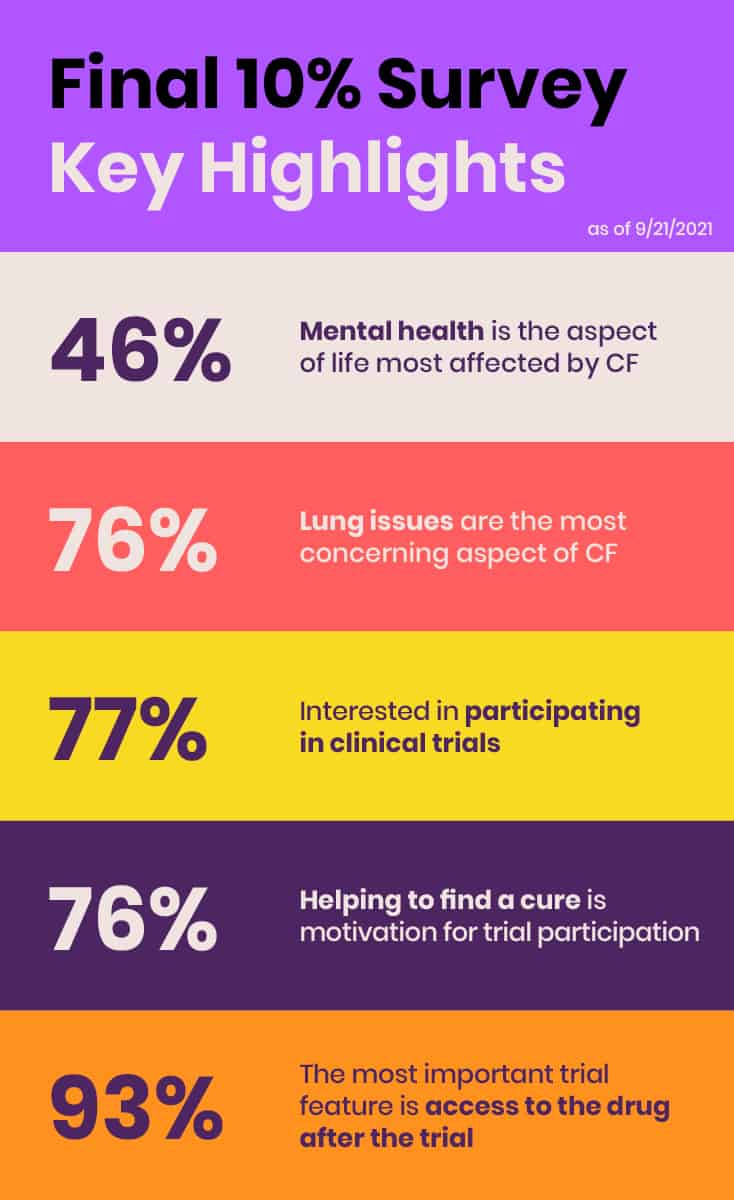
The survey is another example of how EE is identifying needs and filling critical gaps. Although the survey initially was intended to supplement the six CF community members’ stories at the FDA Listening Session with some basic quantitative data, it quickly became clear that its value was much bigger and more expansive. The survey yielded a robust response, with 431 respondents from 29 countries across five continents. EE planned to publish results in a peer-reviewed journal to ensure this data is fully available to the public.



